Volume 1, Year 2014 - Pages 8-15
DOI: 10.11159/jbb.2014.002
Ionic Liquid Pretreatment of Rice Straw to Enhance Saccharification and Bioethanol Production
Nafiseh Poornejad1*, Keikhosro Karimi1,2, Tayebeh Behzad1
1Department of Chemical Engineering, Isfahan University of Technology, Isfahan 84156-83111, Iran
n.poornejad@ce.iut.ac.ir
2Industrial Biotechnology Group, Institute of Biotechnology and Bioengineering, Isfahan University of Technology, Isfahan 84156-83111, Iran
Abstract - In this study, rice straw was pretreated by an ionic liquid, 1-ethyl-3-methylimidazolium acetate ([EMIM][OAc]), prior to enzymatic hydrolysis and ethanol production. The pretreatment was carried out at 120 °C for 5 h under atmospheric pressure. Afterward, saccharification was conducted at 45 °C for 72 h using 20 FPU cellulase and 30 IU β-glucosidase per gram of cellulose. Glucose yield was significantly increased from 25.7% for the untreated straw to over 75% for the treated straw. Moreover, the ethanol yield by simultaneous saccharification and fermentation was improved from 35.6% for the untreated straw to 79.7% for the treated straw. SEM images showed more open and accessible structure for the treated straw compared to the untreated one which assisted the penetration of hydrolytic enzymes in the saccharification and fermentation. From FTIR analysis, it was suggested that the crystallinity reduction and conversion of cellulose I to II are the main changes in the straw as a result of the pretreatment.
Keywords: [EMIM][OAc], Ethanol, Ionic liquid, pretreatment, rice straw.
© Copyright 2014 Authors - This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2013-10-16
Date Accepted: 2013-03-06
Date Published: 2014-05-30
1. Introduction
Recently, lignocellulosic biomasses attract great attention of scientists as a potential feedstock for bioethanol production. Lignocelluloses are widely available in different waste sources such as municipal, agricultural, and forestry residues, and they mainly consist of cellulose, hemicelluloses, and lignin [1]. Cellulose and hemicellulose can be both enzymatically or chemically hydrolyzed to reduced sugar units and further fermented to ethanol. However, complex structure, lignin strong linkage, hydrogen bonds between cellulose chains, and highly crystalline cellulose structure cause these materials to be very resistance to both chemical and enzymatic hydrolysis [1-3]. Therefore, for economically feasible ethanol production, an effective pretreatment step should be applied to improve the saccharification yield.
By now, various chemical, physical, biological, and combined pretreatment methods have been employed. The most common methods are alkali or acid pretreatments [4, 5], ammonia fiber explosion [6], steam or carbon dioxide explosion [7, 8], and liquid hot water pretreatment [9]. However, majority of these methods need high temperatures and pressures or harsh acidic or basic conditions. Therefore, they are not environmentally friendly and require high amount of energy [10]. Recently, to overcome these drawbacks, researchers have attempted to use a new class of cellulose solvents called ionic liquids. Ionic liquids are organic salts that are liquid at room temperature [11]. These materials have some priorities over the conventional solvents including being more environmentally benign, requiring less process time, and being more efficient [12].In addition, the processing conditions of ionic liquids are mild, and their recycling efficiency is over 99% [10]. Cellulose regenerated by ionic liquids is less crystalline and more amenable to enzymatic hydrolysis [13].
The various ionic liquids, 1-Butyl-3-methylimidazolium chloride ([BMIM]Cl), 1-Ethyl-3-methylimidazolium hydroxide ([EMIM]OH), and -ethyl-3-methylimidazolium acetate ([EMIM][OAc]) have superior cellulose solubility characteristic. However, the ionic liquids involving Cl- and OH-are corrosive and can adversely affect the enzymes activity resulting in lower overall hydrolysis yield. [EMIM]][OAc] is among the best cellulose solvents in terms of solubility characterization and compatibility with hydrolytic enzymes. Furthermore, it is an environmentally friendly ionic liquid [14]. Because of these attractive properties, it has been suggested as an effective chemical for lignocelluloses pretreatment to improve hydrolysis and ethanol production [15, 16].
Rice straw is one of the most available lignocelluloses residues in all over the world. Several previous studies have attempted to improve its digestibility to reduced sugar units applying different pretreatment methods such as microwave pretreatment [17, 18], electron beam irradiation [19], and dilute acid hydrolysis [20]. In a recent study, Feng [21] used an ionic solvent, [AMIM]Cl, to treat the straw at 110 ºC. He succeeded to improve the hydrolysis yield from 23.7% for the untreated straw to 53.3% for the pretreated one. However, to our knowledge, no previous work has been done on pretreatment of rice straw by [EMIM][OAc].
The main goal of this study is to enhance bioethanol production of rice straw by applying [EMIM][OAc] pretreatment. Enzymatic hydrolysis and simultaneous saccharification and fermentation were performed to evaluate the pretreatment effect. The compositional and structural properties of the pretreated straw were also studied by different analyses.
2. Materials and Methods
2.1. Materials and Microorganism
The rice straw used in this study was obtained from Lenjan field (Isfahan, Iran). Before experiments, its size was reduced from about 50 mm to less than 0.853 mm (mesh 20) and then dried at 40 ºC for 24 h. The solid content of straw was measured after drying at 105 ºC until a constant weight was achieved.
A commercially available ionic liquid, 1-ethyl-3-methylimidazolium acetate ([EMIM][OAc]), was purchased from Sigma-Aldrich provided by BASF (Ludwigshafen, Germany).
A fluctuation of Saccharomyces cerevisiae (CCUG 53310, Culture Collection of University of Gothenburg, Sweden) was used as a fermenting microorganism. The microorganism maintenance and its biomass production was conducted according to the method presented by Karimi et al. [22].
2.2. Pretreatment Procedure
A suspension of rice straw in the ionic liquid with 5% solid loading were prepared in a 200 ml beaker in an oil bath at 120 ºC and pretreated for 5 h. The pretreatment conditions were selected based on optimized parameters obtained in a previous research [23].The suspension was mixed every 15 min by a glass rode to ensure complete distribution of solid in the solvent. At the end of the pretreatment, the dissolved solid was regenerated by sudden addition of 30 ml boiling water. The precipitated solid was recovered by vacuum filtration and washed by ethanol and boiling water till clear filtrate was achieved. The straw was then dried at 40ºC for three days, and then stored in sealed bags for further use and analysis.
2.3. Enzymatic HydrolysisAME
Cellulase (Celluclast 1.5L, Novozyme, Denmark) and β-glucosidase (Novozyme 188, Novozyme, Denmark) enzymes were used for the hydrolysis. The activity of cellulase was 60 FPU/ml (measured according to Adney and Baker (1996) procedure and that of β-glucosidase was 190 IU/ml (measured by the method presented by Ximenes et al. [24]1996).
Enzymatic hydrolysis of all treated and untreated straws was carried out in 100 ml bottles containing 30 ml media of 50 mM sodium citrate buffer (pH 4.8) and 10 g/l substrate loading. Then 20 FPU cellulase and 30 IU β-glucosidase per gram of substrate and 0.5 g/l sodium azide were added to each bottle. The hydrolysis was conducted in a shaker incubator with the speed of 100 rpm at 45 ºC for 72 h. Liquid samples were periodically taken and analyzed for the released sugar.
2.4. Simultaneous Saccharification and Fermentation (SSF)
A fluctuation of Saccharomyces cerevisiae (CCUG 53310, Culture Collection of University of Gothenburg, Sweden) was used as a fermenting microorganism. The treated and untreated straws were subjected to simultaneous saccharification and fermentation at 38 °C under anaerobic conditions for 48 h in a shaker incubator at 80 rpm. A media containing 5 g/l yeast extract, 7.5 g/l (NH4)2SO4, 3.5 g/l K2HPO4, 0.75 g/l MgSO4·7H2O, 1 g/l CaCl2·2H2O, and 50 g/l pretreated or untreated rice straw was prepared in 0.05 M buffer citrate (pH 5). After sterilization at 121 ºC for 20 min and cooling to room temperature, 1 g/l S. cerevisiae and 20 FPU cellulase and 30 IU β-glucosidase per gram of substrate were added to the mixture and incubated for 48 h. Liquid samples were taken, centrifuged, and analyzed for metabolite and sugar analyses.
The theoretical yield of ethanol production was calculated based on the glucose that can be produced supposedly from the glucan in the treated or non-treated rice straw while the glycerol yield was reported as milligrams of produced glycerol per gram of the theoretical glucose [22].
2.5. Analytical Methods
Ethanol and glycerol contents in the liquid samples obtained by simultaneous saccharification and fermentation as well as various sugar contents (glucose, xylose, arabinose, mannose, and galactose) were analyzed by high-performance liquid chromatography (HPLC) equipped with UV/vis and RI detectors (Jasco International Co., Tokyo, Japan). Ethanol and glycerol were analyzed on an Aminex HPX-87H column (Bio-Rad, Richmond, CA, USA) at 60 °C with 0.6 ml/min 5 mM sulfuric acid as an eluent. In addition, sugars were analyzed on an ion-exchange Aminex HPX-87P column (Bio-Rad, USA) at 85 °C with deionized water as a mobile phase with the flow rate of 0.6 ml/min.
To analyze carbohydrate and lignin contents of the biomass, an standard method presented by National Renewable Energy Laboratory (NREL) was employed [25]. The carbohydrates were hydrolyzed to the corresponding sugars and analyzed by HPLC. Lignin was classified into acid-insoluble and soluble materials. The acid-soluble lignin was measured by UV–vis spectroscopy at 240 nm with absorptivity of 25 l/g.cm. The acid-insoluble part was determined as a part of solid collected by filtration after the hydrolysis.
FTIR reflectance spectra of untreated and treated straws were collected within wave numbers of 600 to 4,000 cm-1 with 4 cm-1 resolution using a Fourier transform infrared (FTIR). The spectrometer was equipped with a universal ATR (Attenuated Total Reflection) accessory and Deuteratedtriglycine sulfate (DTGS) detector (Bruker Tensor 27 FT-IR). Their spectra were obtained with an average of 60 scans.
Scanning electron microscopy (SEM) images of untreated and treated straws were obtained to analyze morphological effect of the pretreatment step. Dried treated and untreated straw were coated with gold (BAL-TEC SCD 005) and analyzed by SEM (PHILIPS, XL30) at 15 kV.
All experiments were done at least in duplicate and the reported results are the average of obtained values. The error bars on reported figures are the range of higher and lower values obtained for each point showing the range of validity of the results.
3. Results and Discussion
Rice straw, one of the most abundant lignocellulosic wastes, was subjected to a solvent pretreatment using [EMIM][OAc] at its optimum treatment conditions which is 120 °C for 5 h [26-28].
3.1. Enzymatic Hydrolysis
In order to examine the effect of pretreatment on rice straw saccharification, enzymatic hydrolysis of all treated and untreated straw was done for 72 h at 45 ºC using cellulase and β-glucosidase enzymes. Glucose and xylose yields of hydrolysis of the treated straw were significantly improved and summarized in Table 1 and Figure 1, respectively.
Table 1. Hydrolysis and fermentation results of pretreated and untreated rice straw.
| Pretreatment | Hydrolysis results (%)2 | Fermentation results3 | |||||||
| After 24h | After 72h | Ethanol yield (%) |
Glycerol yield (mg/g) |
||||||
| Pretreated straw1 | 75.4±2.8 | 90.9±3.2 | 79.7±1.1 | 110.3±4.6 | |||||
| Untreated straw | 23.9±4.6 | 25.7±5.2 | 35.6±0.2 | 57.3±3.1 | |||||
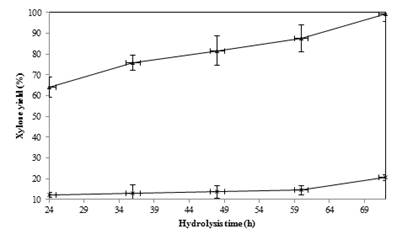
The major hydrolysis was obtained after 24 h hydrolysis, and prolongation of the hydrolysis to 72 h showed a minor improvement (Table 1). Xylose is another product of hydrolysis comes from hemicelluloses hydrolysis. The pretreatment significantly improved the yield of xylan hydrolysis (Figure 1). According to obtained data, hydrolysis rate at the first 24 h was significantly higher for treated straw compared to the untreated one. Moreover, the same trend was observed after 72 h hydrolysis. These results confirm the possibility of the claim that ionic pretreatment step can efficiently improve the straw digestibility in the hydrolysis step.
The results are in line of the results obtained by Shafiei et al. [29] on pretreatment of spruce wood powder by the same ionic liquid. They showed that prolongation of the pretreatment time from 3 h to 15 h did not positively affect the yield of hydrolysis. Furthermore, it was shown that using the pretreatment at high temperature of 120 °C is more effective than the pretreatment with [EMIM][OAc] at lower temperature even for a very long time (i.e., at 90 ºC for 24 or 70 h) [16].
3.2. Simultaneous Saccharification and Fermentation (SSF)
Ethanol was produced by SSF at 38 ºC for 48 h in the presence of cellulase, β-glucosidase, and S. cerevisiae for the untreated and pretreated straw and results are reported in Table 1. As it can be noticed from the data, the ethanol yield was improved from 35% to 79% which indicates the great impacts of the pretreatment. The results of rice straw pretreatment by [EMIM][OAc]are comparable with the results of wheat straw pretreated by [EMIM]DEP [13], a similar ionic liquid with different anionic group. The observed results could be predicted from the fact that the opened up structure and more accessible substrate to the hydrolytic enzymes can have a dramatic positive effect on the digestibility of cellulose which followed by more efficient conversion to the final product.
Glycerol is the major byproduct of ethanol production, and its production improvement by the pretreatment is compatible with the ethanol yield enhancement. Glycerol yield was improved from 57.3 mg/g for untreated straw to 110.3 mg/g of glucose for treated one which shows about 100% improvement.
3.3. Compositional Analysis
To investigate and justify the origin of the observed improvement, the structural composition of treated and untreated straw, supposed to have the most significant importance on hydrolysis, was determined, and the results are shown in Figure 2. As it can be concluded from the results, glucan content of the regenerated solid was increased compared with that of untreated straw which indicates more purified cellulose resulted in more efficient hydrolysis of the pretreated solid. On the other hand, xylan, resulted from the hydrolysis of hemicelluloses, was reduced by the pretreatment. Moreover, lignin, the most important obstacle for enzymatic hydrolysis of lignocellulosic materials, was reduced in precipitated solid in comparison with the untreated straw. Lignin and hemicelluloses are both responsible for recalcitrance of lignocelluloses which were decreased due to solubilization in the ionic liquid and not precipitation during regeneration (addition of the anti-solvent). The hemicellulose and lignin removal leads to a higher concentration and more accessible cellulose, which improved the yield of hydrolysis and ethanol production. There is also a minor change in ash content of the straw as a result of the pretreatment.
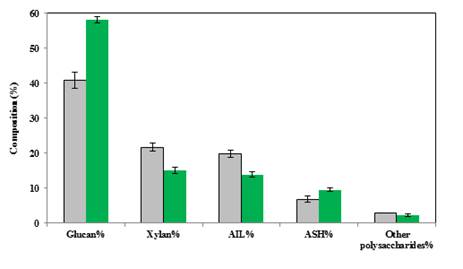
As discussed in the hydrolysis result section, it could be predicted that increasing pretreatment retention time at high temperature (more than 100 ºC) may cause some degradation effect on cellulose and hemicelluloses; however, cellulose percent is still increased. The degradation of glucan and xylan at 130 ºC was proved by Lee et al. [16]. In addition, the lignin removal effect of ionic liquid treatment was also observed by several previous studies [16, 30-32].
3.4. Change in the Morphology of Rice Straw by Pretreatment
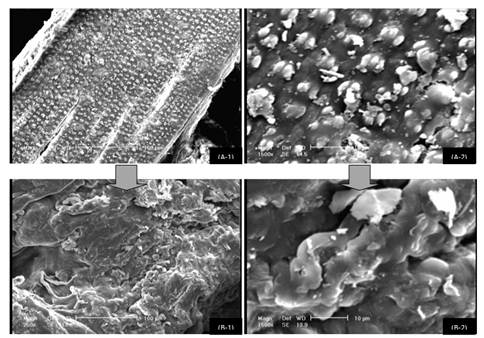
Morphology of the pretreated and untreated straw was analyzed by SEM images (Figure 3). One of the main obstacles, or maybe the most effective one, for the digestibility of straw is crystalline and organized structure of the cellulosic fibers. As could be seen in SEM images, the organized structure of the straw is completely destroyed as a result of the pretreatment. Therefore, the structure of the straw was changed from a compact and unavailable form to an open up and widely accessible form. This could assist and accelerate the penetration of the enzymes to the carbohydrates. Also, there are some fine spots on the untreated straw which disappear on the treated one. These spots are silica which is one of the problematic component of rice straw in both its storage and conversion. Removal of this component as a result of the pretreatment is another possible reason for improvement of enzymatic hydrolysis.
To evaluate the pretreatment effect on cellulose crystallinity of straw, FTIR analysis of the treated and untreated straw was conducted. FTIR spectra and absorbance data are presented in Figure 4 and Table 2, respectively.
Table 2. Characteristic and variation of bands in FTIR spectra of the untreated rice straw and the straw treated with [EMIM][OAc] for 5 h at 120 ºC.
| Wavenumber (cm-1) |
Functional group | Assignment | Untreated straw | Treated straw for 5 h |
| 3175 | –OH stretching intramolecular hydrogen bonds | Cellulose II | 0.1 | 0.17 |
| 2918 | C–H stretching | Cellulose | 0.11 | 0.16 |
| 1730 | C=O stretching of acetyl or carboxylic acid | Hemicellulose & lignin | 0.07 | 0.05 |
| 1627 | C=C stretching of the aromatic ring | Lignin | 0.17 | 0.12 |
| 1598 | C=C | Lignin | 0.14 | 0.09 |
| 1510 | C=C stretching of the aromatic ring | Lignin | 0.1 | 0.1 |
| 1465 | Asymmetric bending in C–H3 | Lignin | 0.13 | 0.11 |
| 1423 | C–H2 symmetric bending | Cellulose | 0.17 | 0.2 |
| 1430 | C–H2 bending | Cellulose I | 0.17 | 0.19 |
| 1375 | C–H bending | Cellulose | 0.18 | 0.24 |
| 1335 | –OH (in plane bending) | Cellulose | 0.18 | 0.21 |
| 1315 | C–H2 wagging | Cellulose | 0.21 | 0.22 |
| 1158 | C–O–C asymmetric stretching | Cellulose | 0.42 | 0.39 |
| 896 | Asym., out of phase ring stretching (cellulose) | Cellulose II | 0.36 | 0.49 |
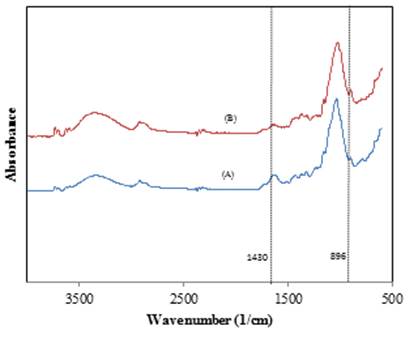
The absorption bands at 1430 cm-1 represents crystalline cellulose I, while the band at 898 cm-1 is assigned to the cellulose II and amorphous cellulose which is more amenable to enzymatic hydrolysis [33]. The results showed that cellulose I in the native straw was changed to cellulose II as a result of the pretreatment. Furthermore, the absorption ratio at absorption bands of 1430 and 896 cm-1, referred to as crystallinity index (CI) [33], was used for evaluation of crystallinity reduction due to the pretreatment. This value for untreated straw and treated straw was 0.47 and 0.38, respectively. Moreover, another index for crystallinity evaluation is total crystallinity index (TCI), the ratio of absorption bands at 1975 and 2918 cm-1, was obtained to be 1.6 and 1.5 for the untreated and treated straws, respectively. As indicated by both CI and TCI calculations, majority of crystalline cellulose was modified to amorphous and less crystalline regions which are amenable to enzymatic attack.
3.5. Mass Balance
Overall mass balance for pretreatment, hydrolysis, and simultaneous saccharification and fermentation step was presented in Table 3. As stated by the observed data, each ton of pretreated rice straw can produce 450 kg glucose and 250 L ethanol. However, these values for untreated straw are only 120 kg and 100 L, respectively. Reported by FAO, the worldwide rice production in 2012 was 724.5 million tons. On average, 1-1.5 kg straw is produced per kg of rice [34] which results in production of 900 million tons rice straw annually. According to the results of current study, this amount of straw has a potential to produce 225 billion liters ethanol per year.
Table 3. Overall mass balance over pretreatment, hydrolysis, and simultaneous saccharification and fermentation for untreated and pretreated rice straw.
| Substrate | Untreated straw | Pretreated straw |
| Grams of solid recovered after pretreatment per 100 grams of initial straw | - | 73±2.1 |
| Grams of glucose obtained after 72 h hydrolysis per 100 grams of initial straw | 11.7±1.8 | 44.3±3.9 |
| Grams of ethanol released after 48 h SSF per 100 grams of initial straw | 8.3±1.3 | 19.2±2.4 |
4. Conclusions
Pretreatment of rice straw using ionic liquid [EMIM][OAc] can be considered as a promising alternative to improve the enzymatic digestibility and ethanol production. Pretreatment of rice straw at 120 ºC for 5 h can improve the yield of ethanol production by 75 %. More accessible morphology, less crystallinity, lower lignin and hemicelluloses concentrations are the main reasons for the improvements.
Acknowledgment
The authors are grateful to Institute of Biotechnology and Bioengineering, Isfahan University of Technology for financial support of this work.
References
[1] M.J. Taherzadeh and K. Karimi, "Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review", International Journal of Molecular Sciences, 9(9), 2008, 1621-1651. View Article
[2] A.T.W.M. Hendriks and G. Zeeman, "Pretreatments to enhance the digestibility of lignocellulosic biomass" Bioresource Technology, 100(1), 2009, 10-18. View Article
[3] N. Mosier, Ch. Wyman, B. Dale, R. Elander, Y.Y. Lee, M. Holtzapple, M. Ladisch, "Features of promising technologies for pretreatment of lignocellulosic biomass", Bioresource Technology, 96(6), 2005, 673-86. View Article
[4] Z. Hu and Z. Wen, "Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment", Biochemical Engineering Journal, 38(3), 2008, 369-378. View Article
[5] B.C. Saha, L.B. Iten, M.A. Cotta, V. Wu, "Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol", Process Biochemistry, 40(12), 2005, 3693-3700. View Article
[6] H. Alizadeh, F. Teymouri, T. Gilbert, B. Dale, "Pretreatment of switchgrass by ammonia fiber explosion (AFEX)", Applied Biochemistry and Biotechnology, 124(1-3), 2005, 1133-1141. View Article
[7] M.J. Negro, P. Manzanares, J.M. Olivia, I. Ballesteros, M. Ballesteros, "Changes in various physical/chemical parameters of Pinus pinaster wood after steam explosion pretreatment", Biomass and Bioenergy, 25(3), 2003, 301-308. View Article
[8] V.P. Puri and H. Mamers, "Explosive pretreatment of lignocellulosic residues with high-pressure carbon dioxide for the production of fermentation substrates", Biotechnology and Bioengineering, 25(12), 1983, 3149-3161. View Article
[9] Q. Yu, Y. Zhuang, Q. Wang, W. Qi, W. Wang, Y. Zhang, J. Xu, H. Xu, "Two-step liquid hot water pretreatment of Eucalyptus grandis to enhance sugar recovery and enzymatic digestibility of cellulose", Bioresource Technology, 101(13), 2010, 4895-4899. View Article
[10] P. Mäki-Arvela, I. Anugwom, R. Sjoholm, J.P. Mikkola, "Dissolution of lignocellulosic materials and its constituents using ionic liquids-A review", Industrial Crops and Products, 32(3), 2010, 175-201. View Article
[11] H. Zhao, C. Jones, G. Baker, Sh. Xia, O. Olubajo, V.N. Person, "Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis", Journal of Biotechnology, 139(1), 2009, 47-54. View Article
[12] D. Fu and G. Mazza, "Aqueous ionic liquid pretreatment of straw", Bioresource Technology, 102(13), 2011, 7008-7011. View Article
[13] Q. Li, B. Knierima, Ch. Manisseri, R. Arora, H.V. Scheller, M. Auer, K.P. Vogel, A. Simmons, S. Singh "Improving enzymatic hydrolysis of wheat straw using ionic liquid 1-ethyl-3-methyl imidazolium diethyl phosphate pretreatment", Bioresource Technology, 100(14), 2009, 3570-3575. View Article
[14] L. Li, S-T. Yu, F-S. Liu, C-X. Xie, C-Z. Xu "Efficient enzymatic in situ saccharification of cellulose in aqueous-ionic liquid media by microwave pretreatment", Bioresources, 6(4), 2011, 11. View Article
[15] T.N. Ang, L.W. Yoon, K.M. Lee, G.C. Ngoh, A.S.M. Chua, M.G. Lee, "Efficiency of ionic liquids in the dissolution of rice husk", Bioresources, 6(4), 2011, 10. View Article
[16] S.H. Lee, Th. V. Dohrety, R.J. Linhardt, J.S. Dordik, "Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis", Biotechnology and Bioengineering, 102(5), 2009, 1368-1376. View Article
[17] S. Zhu, Y. Wu, Z. Yu, C. Wang, F. Yu, S. Jin, Y. Ding, R. Chi, J. Liao, Y. Zhang, "Comparison of three microwave/chemical pretreatment processes for enzymatic hydrolysis of rice straw", Biosystems Engineering, 93(3), 2006, 279-283. View Article
[18] G. Gong, D. Liu, and Y. Huang, "Microwave-assisted organic acid pretreatment for enzymatic hydrolysis of rice straw", Biosystems Engineering, 107(2), 2010, 67-73. View Article
[19] J.S. Bak, J.K. Ko, Y. Han, B.Ch. Lee, I. Choi, K. Kim, "Improved enzymatic hydrolysis yield of rice straw using electron beam irradiation pretreatment", Bioresource Technology, 100(3), 2009, 1285-1290. View Article
[20] K. Karimi, S. Kheradmandinia, and M.J. Taherzadeh, "Conversion of rice straw to sugars by dilute-acid hydrolysis", Biomass & Bioenergy, 30(3), 2006, 247-253. View Article
[21] H.S. Hong Feng, "Biorefinery of bacterial cellulose from rice straw: enhanced enzymatic saccharification by ionic liquid pretreatment", Engineering science, 9(4), 2011, 4. View Article
[22] K. Karimi, G. Emtiazi, and M.J. Taherzadeh, "Ethanol production from dilute-acid pretreated rice straw by simultaneous saccharification and fermentation with Mucor indicus, Rhizopus oryzae, and Saccharomyces cerevisiae", Enzyme and Microbial Technology, 40(1), 2006, 138-144. View Article
[23] N. Poornejad, "Improved Saccharification and Fermentation of Rice Straw using Various Solvents for Pretreatment Step", in Chemcial Engineering, Isfahan University of Technology: Isfahan, 2012, p. 90.
[24] E.A. Ximenes, C.R. Felix, and C.J. Ulhoa, "Production of cellulases by Aspergillus fumigatus and characterization of one b-glucosidase", Current Microbiology, 32(3), 1996, 119-23. View Article
[25] A. Sluiter, B. Hames, C. Ruiz, J. Sluiter, D. Templeton, D. Crocker, "Determination of Structural Carbohydrates and Lignin in Biomass", Laboratory Analytical Procedure, NREL/TP-510-42618, 2008. View Article
[26] H. Xie, S. Li, S. Zhang, "Ionic liquids as novel solvents for the dissolution and blending of wool keratin fibers", Green Chemistry, 7(7), 2005, 3. View Article
[27] A.P. Dadi, S. Varanasi, and C.A. Schall, "Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step", Biotechnology Bioengineering, 95(5), 2006, 904-10. View Article
[28] J. Wu, J. Zhang, H. Zhang, J. He, Q. Ren, M. Gue, "Homogeneous Acetylation of Cellulose in a New Ionic Liquid", Biomacromolecules, 5(2), 2004, 266-268. View Article
[29] M. Shafiei, H. Zilouei, A. Zamani, M.J. Taherzafeh, K. Karimi, "Enhancement of ethanol production from spruce wood chips by ionic liquid pretreatment", Applied Energy, 102, 2013, 163-169. View Article
[30] D.A. Fort, R.C. Remsing, R.P. Swatloski, P. Moyna, G. Moyna, R.D. Rogers, "Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride", Green Chemistry. 9(1), 2007, 63-69. View Article
[31] J.B. Binder, M.J. Gray, J.F. White, C. Zhang, J.E. Holladay, "Reactions of lignin model compounds in ionic liquids", Biomass and Bioenergy, 33(9), 2009, 1122-1130. View Article
[32] N. Sun, M. Rahman, M.L. Maxim, H. Rodriguez, R.D. Rogers, "Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate", Green Chemistry, 11(5), 2009, 646-655. View Article
[33] D.C. Nieves, K. Karimi, and I.S. Horváth, "Improvement of biogas production from oil palm empty fruit bunches (OPEFB)", Industrial Crops and Products, 34(1), 2011, 1097-1101. View Article
[34] P. Binod, N. Kurien, R.K. Sukumaran, A. Pandey, "Bioethanol production from rice straw: An overview", Bioresource Technology, 101(13), 2010, 4767-74. View Article

